Researchers Crack Mystery of Swirling Vortexes in Egg Cells

Egg cells are the largest single cells on the planet. Their size — often several to hundreds of times the size of a typical cell — allows them to grow into entire organisms, but it also makes it difficult to transport nutrients and other molecules around the cell. Scientists have long known that maturing egg cells, called oocytes, generate internal, twister-like fluid flows to transport nutrients, but how those flows arise in the first place has been a mystery.
Now, research led by computational scientists at the Flatiron Institute, along with collaborators at Princeton and Northwestern universities, has revealed that these flows — which look like microscopic tornados — arise organically from the interactions of a few cellular components. Their work, published in the April issue of Nature Physics, used theory, advanced computer modeling, and experiments with fruit fly egg cells to uncover the twisters’ mechanics. The results are helping scientists better understand foundational questions about egg cell development and cellular transport.
“Our findings represent a big leap in this field,” says co-author Michael Shelley, director of the Flatiron Institute’s Center for Computational Biology (CCB). “We were able to apply advanced numerical techniques from other research that we’ve been developing for years, which allow us a much better look at this issue than has ever been possible before.”
In a typical human cell, it takes only 10 to 15 seconds for a typical protein molecule to meander from one side of the cell to the other via diffusion; in a small bacterial cell, this trip can happen in just a single second. But in the fruit fly egg cells studied here, diffusion alone would take an entire day — much too long for the cell to function properly. Instead, these egg cells have developed ‘twister flows’ that circle around the interior of the oocyte to distribute proteins and nutrients quickly, just as a tornado can pick up and move material much farther and quicker than wind alone.
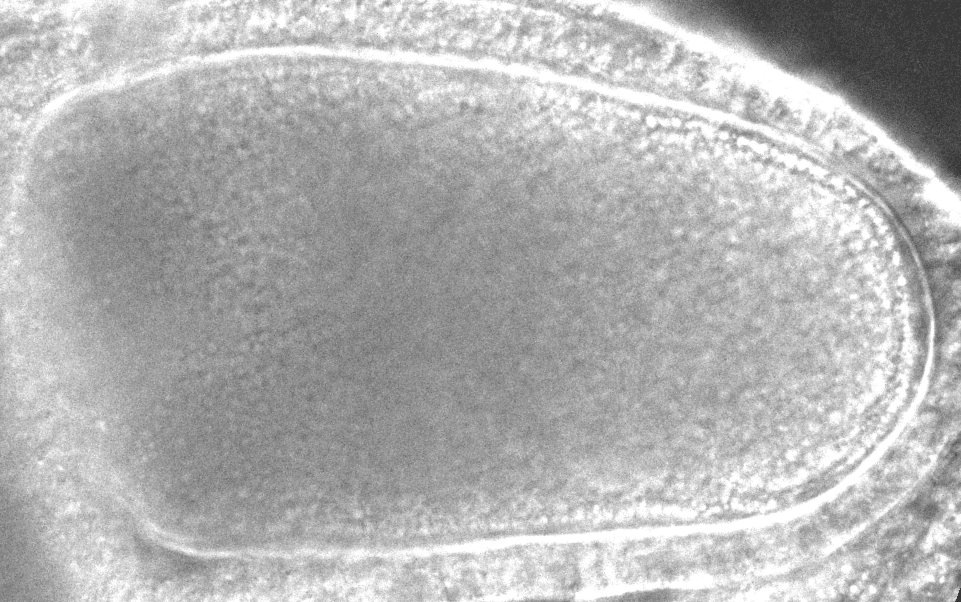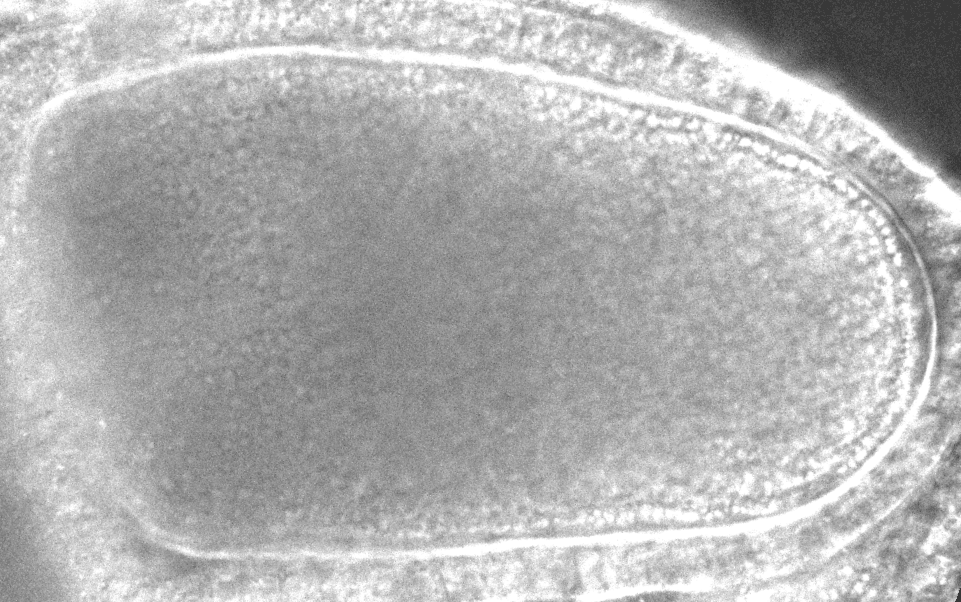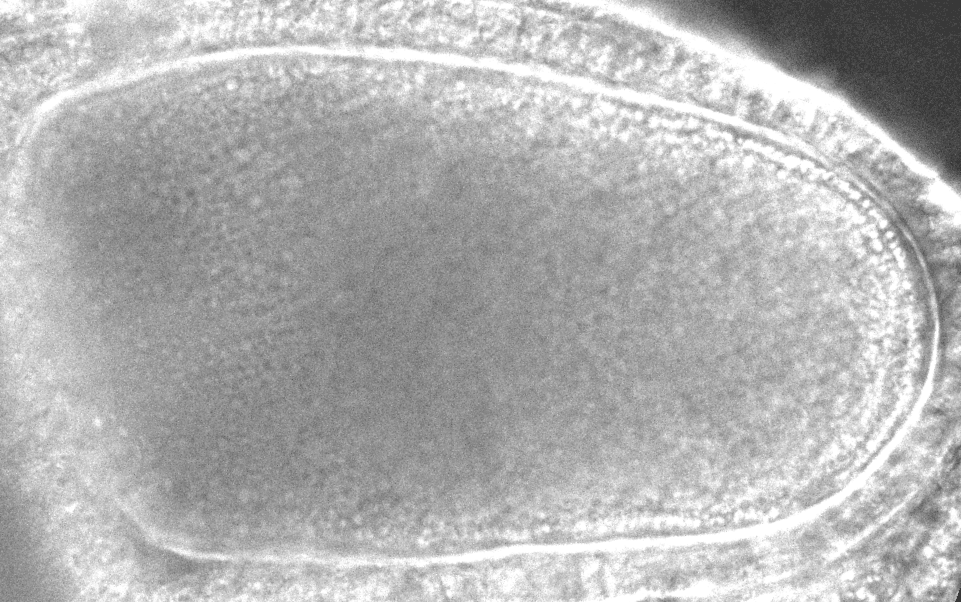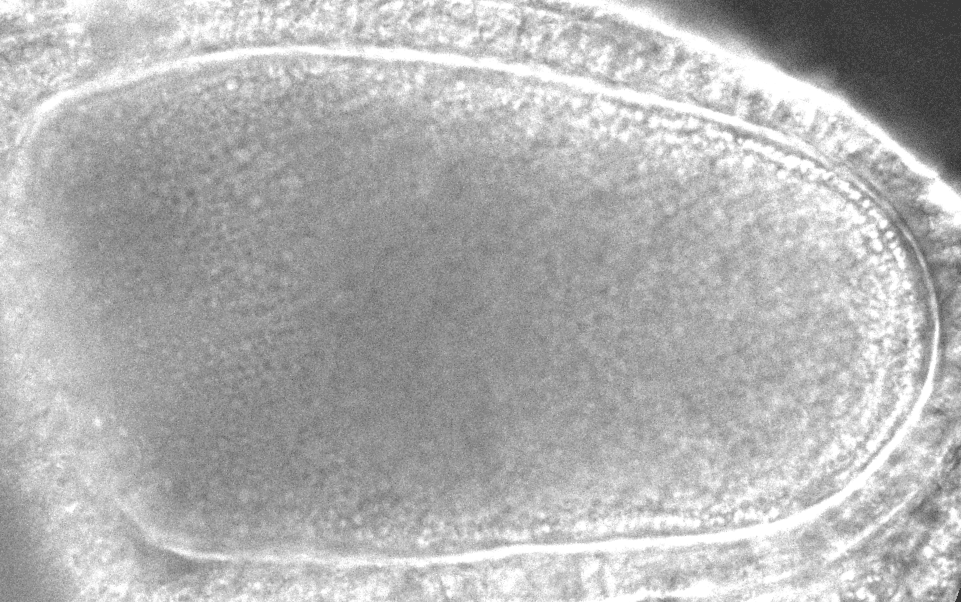
“After it’s fertilized, the oocyte will become the future animal,” says study co-author Sayantan Dutta, a researcher at Princeton and the CCB. “If you destroy the flow in the oocyte, the resulting embryo doesn’t develop.”
The researchers used an advanced open-source biophysics software package called SkellySim developed by Flatiron Institute researchers. With SkellySim, they modeled the cellular components involved in creating the twisters. These include microtubules — flexible filaments that line the inside of a cell — and molecular motors, which are specialized proteins that serve as cellular workhorses, carrying special groups of molecules known as payloads. Scientists aren’t exactly sure what these payloads are made of, but they play a key role in generating the flows.
The researchers simulated the motion of thousands of microtubules as they responded to the forces exerted by payload-carrying molecular motors. By going back and forth between experiments and their simulations, the researchers were able to understand the structure of the twister flows and how they arose from the interaction between the cellular fluid and microtubules.
“Our theoretical work allows us to zoom in and actually measure and visualize these twisters in 3D,” says study co-author and CCB research scientist Reza Farhadifar. “We saw how these microtubules can generate large-scale flows just through self-organization, without any external cues.”
The models revealed that inside the oocyte, microtubules buckle under the force of the molecular motors. When a microtubule buckles or bends under this load, it causes the surrounding fluid to move, which can reorient other microtubules. In a large enough group of bending microtubules, all the microtubules bend in the same direction, and the fluid flows become ‘cooperative.’ With the microtubules collectively bent, the moving payloads create a whirlpool or twister-like flow across the whole egg, helping molecules disperse around the cell. With the twisters, molecules can travel across the cell in 20 minutes instead of 20 hours.
“The model showed the system has an incredible capacity for organizing itself to create this functional flow,” Shelley says. “And you just need a few ingredients — only microtubules, the geometry of the cell, and molecular motors carrying payloads.”
The new findings lay the foundation for a better understanding of egg cell development. The results could also help demystify material transport in other cell types.
“Now that we know how these twisters form, we can ask deeper questions, like how do they mix the molecules inside the cell?” Farhadifar says. “This opens a new dialogue between theory and experiment.”
The new work provides a fresh look at microtubules, Dutta says. Microtubules play a central role in various cell types and cell functions — such as cell division — across almost all eukaryotic organisms, such as plants and animals. That makes them “a very important part of a cell’s toolbox,” Dutta says. “In better understanding their mechanisms, I think our model will help drive the development in a lot of other really interesting problems in cellular biophysics.”
Information for Press
For more information, please contact Stacey Greenebaum at press@simonsfoundation.org.


