
A ‘Forest Fire’ Model of Cell Division Can Explain Clonal Dominance in Developing Tissue
In a burning forest, fire moves quickly but unevenly, destroying entire sections of forest while leaving others untouched. A similar phenomenon occurs with a fast-moving viral infection as it spreads through a community, devastating some households while sparing others. In certain biological systems, yet another pattern of unequal spread occurs. This inequality, called clonal dominance, occurs when some cells multiply in greater numbers than others. The result is that most descendants will be derived from just a small portion of founding cells. Increasingly observed by researchers in recent years, clonal dominance is seen as key to unraveling many mysteries of developmental biology. And now, a team of computational biologists has developed a mathematical model that explains the mechanism by which clonal dominance can arise in the fruit fly’s egg chamber, where dividing cells are able to induce some — and only some — of their neighboring cells to divide. They report their findings in the November issue of Nature Physics.
“The founding clusters of cells expanded in numbers unequally, and we wanted to find the simplest way that could happen,” says Stanislav Shvartsman, head of the developmental dynamics group at the Flatiron Institute’s Center for Computational Biology (CCB), a professor at Princeton University and the paper’s corresponding author. “We show that clonal dominance can happen by cell communication, rather than any inherent advantage within some founder cells, as has been demonstrated in other systems.”
Clonal dominance has been observed in several biological systems, from the developing zebrafish heart to the mouse liver. But it is notoriously challenging to study, as cell descendants in these complex animal models are difficult to track fully over time. In the fruit fly, the egg chamber epithelium — the single layer of cells encasing the egg chamber — offers several advantages for studying clonal dominance. As cells in the epithelium divide, the resulting daughter cells do not separate completely. Rather, they remain connected through cellular bridges called ring canals. This means that a cell’s descendants, called clones, form a cluster of interconnected cells. These clusters serve as a record of prior cell divisions — like a family tree.
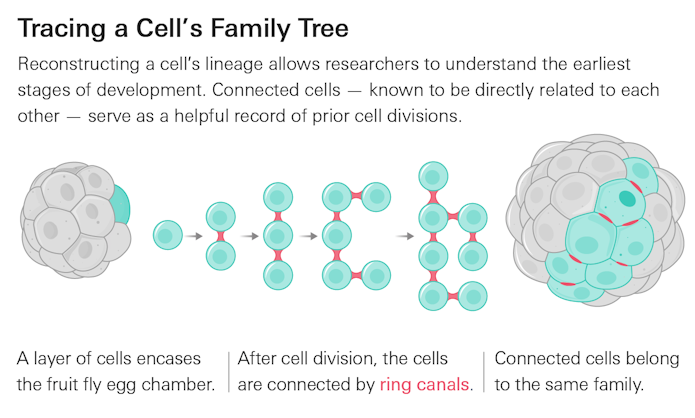
Using the fluorescent marker protein Pavarotti, which binds to epithelium ring canals, the researchers were able to trace cell division histories in fruit fly egg chamber epithelia. The marker lit up the ring canals so a whole epithelium looked “like a disco ball. We could see clusters of related cells,” says Jasmin Imran Alsous, a research scientist in the developmental dynamics group at the CCB and the paper’s co-first author. By poring over epithelium images and counting the sizes of connected cell clusters, Imran Alsous found that the early stages of development — when there were around 30-50 cells — had a dozen or so clusters of between two and eight connected cells and roughly 10 unconnected cells. But by the later stages, when the epithelium contained close to 1,000 cells, one or two dominant clusters had emerged, with the largest clusters containing between 30 and 40 percent of all epithelial cells. “The inequality was striking,” says Shvartsman.
At first glance, these dominant clusters did not appear to have other distinguishing characteristics. For example, the clusters showed no apparent pattern or orientation, and the sizes of the cells in these clusters were the same as those of the nondominant epithelial cells. “The cells in the dominant clusters appeared to maintain their uniform size even though they divide more often,” says Imran Alsous.
To characterize the inequality in epithelial cells, Shvartsman turned to an unlikely source: economics. In economics, the Gini coefficient is used to describe wealth inequality in a population with an equation that examines pairwise differences in wealth among individuals. For this new study, the researchers applied the Gini coefficient equation to the unequal cell distribution among the clusters in the epithelium. With coefficient values that described the inequality seen in the early and late stages of the developing egg chamber, the researchers could test various theoretical models to propose a mechanism behind the observed clonal dominance.
Based on prior studies, the team hypothesized that ring canals held the key to such a mechanism. Previous research had shown that ring canals are large enough to allow proteins to move between connected cells, and that divisions take place in adjacent cells more often than would be expected if the process were random and independent. Therefore, it seemed possible that the diffusion of cell division-promoting factors between linked cells could ‘couple’ their divisions, so that one dividing cell can also induce some of its neighbors to divide, thereby leading to clonal dominance.
To test this hypothesis, the researchers developed a modified version of the well-known ‘forest fire’ model, which showed robust agreement with both the experimental Gini coefficients and cell cluster sizes. The model represented the complete network of linked cells, with individual cells in either a tree state (the cell can divide but isn’t currently doing so), a fire state (it is dividing), or a refractory, or resting, state (the cell is not yet ready to divide following a prior division). Cells can either divide spontaneously or be induced to divide by a linked dividing cell, a process reminiscent of the way fire spreads between adjacent trees. “Here is a very simple model that captures the experimental results well without a lot of fine-tuning,” says Jan Rozman, a research assistant at the Jožef Stefan Institute and a doctoral student at the University of Ljubljana in Slovenia, and the paper’s co-first author.
Réka Albert, a professor of physics and biology at Pennsylvania State University who was not involved in the study, agrees. “This work implies that a cell’s response to a signal depends both on the strength of the signal and the internal state of the cell. This is a very interesting departure from the usual concept of signal transduction,” she says.
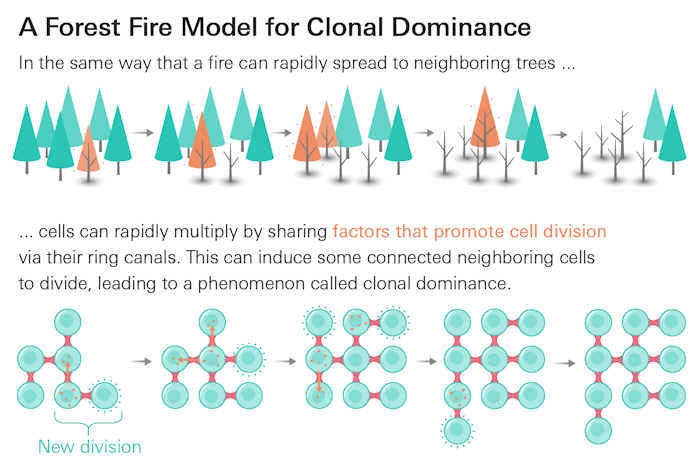
Indeed, this model shows that clonal dominance can arise spontaneously, without any special founder advantage, as long as the cells are communicating with each other. The idea of signal propagation throughout a system where the parts have a resting state is a new framework for understanding cell division, says Stefano Di Talia, an associate professor of cell biology at the Duke University School of Medicine who was not involved in the study.
“I think this work perhaps raises more questions than it answers,” says Imran Alsous. Shvartsman adds, “This model sharpens our view of this system and shines light on what we don’t yet understand.” In particular, Shvartsman says this research leads the team to want to better understand how the cells know when to stop dividing once the epithelium contains 1,000 cells, and the nature and impact of the forces the cells exert on each other. “This system contains many more secrets that we would like to uncover,” he says.


