Searching for the Secret of How Young Blood Rejuvenates the Brain

Vampire legends posit that the blood of a young person can restore youth to the old, a treatment that has lured in wealthy seniors from medieval popes to Korean dictator Kim Il-Sung. But the legendary power of young blood is not simple fiction. It also appears to have a strong basis in biology.
Growing evidence over the last 20 years reveals numerous benefits. Using a procedure called parabiosis, in which two mice are joined together so that they share a circulatory system, scientists have shown that old mice that receive blood from younger animals can run faster and longer on a treadmill than their untreated counterparts. The treated mice also learn more quickly how to avoid an electrical shock and remember better how to swim through a water maze. Their brains generate new neurons in the hippocampus — the region of the brain that controls learning and memory. Young blood, it seems, is one of the most powerful anti-aging treatments — at least in mice.
Translating these findings into humans, however, is challenging. Simply transfusing blood from young donors into older people is not a viable therapeutic option: The process carries a high risk of infection and is impossible to standardize from patient to patient.
In an ideal world, scientists would isolate a specific factor in young blood that reverses the cognitive decline and memory loss that occurs with aging. Over the last decade or so, researchers have found a handful of potential candidates. But it has also become clear that no single protein is responsible for the benefits of young blood — none of these individual factors is as powerful in reversing aging as parabiosis itself. “I think we have a very rudimentary understanding at this point of how [parabiosis] works,” says Tony Wyss-Coray, a neurologist at Stanford University and an investigator with the Simons Collaboration on Plasticity and the Aging Brain (SCPAB).
Indeed, the process may involve more than simply adding a key ingredient. Some research suggests that removing factors from old blood may be just as important as adding factors from young blood. Age-related changes in the brain’s immune system and the barrier that regulates which proteins go in and out of the brain further complicate the picture. “I really think we’re not going to find some magic gene where if you fix this one system and put a Band-Aid on it, it’ll make all the difference,” says Jennifer Garrison, a molecular biologist at the Buck Institute for Research on Aging in Novato, California. Instead, the fix will require multiple proteins and other blood factors, as well as a better understanding of the body’s physiology.
Hunting for the magic protein
For a brief moment in 2014, it seemed that researchers might have found the magic blood protein that could explain the effects of parabiosis. Lee Rubin, a stem cell biologist at Harvard University and a SCPAB investigator, and his colleagues zeroed in on a protein called GDF11, which some evidence suggests is prevalent in young blood but decreases with age. Injecting GDF11 into old mice, they found, could improve blood flow in the brain and even spark the growth of new neurons in the hippocampus.
Rubin says that GDF11 is probably one of multiple proteins in young blood that lead to rejuvenation. He and others have identified about half a dozen blood proteins that are elevated in young animals’ blood and seem to play a role in brain aging. They include TIMP2, a protein found in umbilical cord blood, and klotho, a hormone produced in the kidneys. In some cases, injecting these proteins into old mice can enhance the growth of new neurons, regenerate muscles, and generally make the mice appear younger than their elderly counterparts.
Meanwhile, other researchers have found a number of harmful blood proteins that increase with age. Injecting them into young mice quickly causes cognitive problems and muscle degeneration. Bioengineer Irina Conboy of the University of California, Berkeley, for instance, thinks that the old factors overwhelm the young ones. In a recent paper, her group removed half of an old mouse’s blood plasma and replaced it with saline. The method, they found, was as effective at restoring brain and muscle function as replacing it with young blood. In fact, some of the proteins that are prevalent in young blood reappeared as soon as the old blood was diluted, suggesting they’d been suppressed by the old blood.
This plasma dilution system, known as plasmapheresis, is already used to treat autoimmune conditions such as multiple sclerosis and lupus, and Conboy believes a version of this treatment could be used as an anti-aging therapy as well. Earlier this year, her team started a small clinical trial in which they diluted old patients’ blood with saline, and she expects results early next year. “You can’t completely suppress [old blood proteins] because there is no such thing as a bad protein,” she says. “All proteins are evolved to do some important things in our bodies. What happens is that they just become excessive.”

It’s not only youth that confers anti-aging power on blood. Exercise — a well-established way to protect against the cognitive effects of aging — seems to have similar benefits. In a July paper published in Science, Saul Villeda, a neuroscientist at the University of California, San Francisco and an SCPAB investigator, gave a group of sedentary old mice transfusions of blood from old mice that regularly exercised. After three weeks, the sedentary mice started performing significantly better on learning and memory tests and produced more neurons in their brains. The researchers found that the exercising mice produced more of a blood protein called GPLD1, which delivered the same benefit as exercise when injected into sedentary mice.
The finding that exercise improves cognition regardless of aging suggests that effective therapies with anti-aging proteins might require a combination of treatments. “It could be as simple as blocking an aging factor with exercise and promoting regeneration with a couple factors in young blood,” Villeda says. “Once you can get down to the molecules, you can make them.”
Ultimately, Rubin says, finding the right cocktail will come down to finding the right ratio of beneficial young factors and inhibitors of harmful old factors. “You can find single factors that make stuff better; you can find single factors that make stuff worse,” he says. “Now it’s a question of understanding what those factors are and what they do and what works best with fewest side effects.”
Physiological complications
Understanding the constellation of pro- and anti-aging blood proteins has proved particularly vexing because they appear to work through two of the body’s most complex systems: the immune system and the brain. GDF11, klotho, GPLD1 and several other promising candidate blood proteins can’t cross into the brain themselves — yet they have profound effects on the brain. For example, they appear to improve learning and memory by stimulating the hippocampus to produce new neurons. Researchers suspect that these blood factors activate other proteins in the periphery that can enter the brain or communicate with the neural immune cells that maintain the barrier between the brain and the rest of the body.
Scientists know surprisingly little about the blood-brain barrier, the layer of vascular and endothelial cells that regulates what goes in and out of the brain. Previous research has shown that the barrier tends to break down as people grow older, suggesting an important role in aging. New findings published in Nature in July help explain how. Using a new method for tracking the movement of hundreds of proteins into the brain, Wyss-Coray’s group found that in young mice, cells in the blood-brain barrier used specialized receptors to recognize these proteins and actively transport them into the brain. In old mice, however, unwanted proteins flowed into the brain due to changes in the blood-brain barrier.
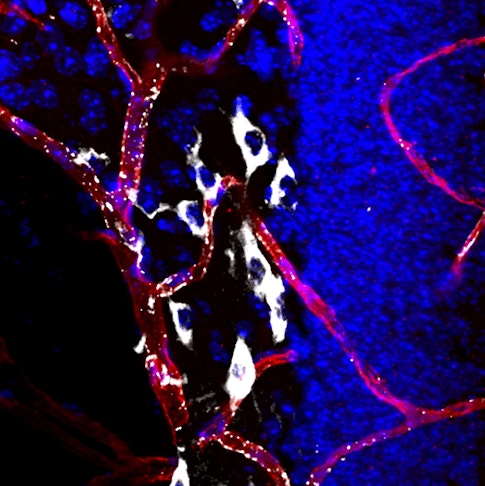
The blood-brain barrier could be a good target for anti-aging drugs, Wyss-Coray says. His group tested a compound called levamisole, which is used clinically to combat hookworm. Levamisole blocks one of the enzymes that allow proteins to freely pass the blood-brain barrier in old mice. Old animals that received the drug began transporting proteins more selectively, as young mice do. Wyss-Coray says that levamisole, or a derivative of it, might one day be used to restore proper function to the blood-brain barrier in aging people.
Wyss-Coray and Rubin are now studying gene expression in single cells within the blood-brain barrier, as well as in other cell types, to see how they change during parabiosis. Any differences in gene expression might provide clues to the secondary effects of blood proteins, ultimately revealing how they get their instructions into the brain.
Findings like Wyss-Coray’s suggest that the blood-brain barrier could be more interesting than previously thought. “For decades, the blood-brain barrier was just this obstacle for how to get drugs into the central nervous system,” says Daniela Kaufer, a molecular biologist at the University of California, Berkeley. Now it is a target itself, she says, “a newborn field.”
Kaufer’s own research has shown that the breakdown in the blood-brain barrier that occurs with age allows a blood protein called albumin to enter the brain and trigger inflammation. When her group injected old mice with a drug to restore the blood-brain barrier and block inflammation, they found that the animals’ scores on memory and learning tests improved significantly.
These results, which were published in Science Translational Medicine in December, are exciting because they suggest that age-related cognitive problems can be reversed. These problems could be caused by an inflammatory “fog” that prevents the brain from working correctly, Kaufer says, rather than a massive die-off of brain cells. “When you remove that inflammatory fog, the capacity for plasticity and function is still there,” she says.
Translating to humans
Translating these advances into treatments that slow aging in humans could be challenging not just because of scientific hurdles but because of regulatory ones. Most drugs in clinical development are designed to treat specific age-related disorders, such as Alzheimer’s or Parkinson’s disease, rather than to slow a natural process. The U.S. Food and Drug Administration (FDA) considers aging to be normal, not a disease that can be treated with FDA-approved therapies. That’s frustrating to some researchers. “I’m fascinated by the fact that everyone accepts loss of cognitive ability as normal,” Rubin says. “Why should we call that normal?”
The FDA regulations governing blood transfusions are somewhat fuzzier. Because transfusions aren’t restricted to treating specific diseases, physicians can use their own judgment in determining when to use them. In recent years, a number of companies have sprung up offering transfusions of young blood as an anti-aging treatment for as much as $8,000. These therapies have not been clinically tested, and the FDA has spoken out against the use of young blood and plasma for this purpose because of the lack of standardized treatments and the danger of infection.
Drug developers hope that data from dementia trials and the development of these drugs will ultimately provide a path for getting anti-aging therapies approved. Because aging is the biggest risk factor for diseases like Alzheimer’s and cancer, treatments that prevent key aspects of aging could also prevent these diseases, says Steven Braithwaite, chief scientific officer of the biotech company Alkahest. Braithwaite and others are using ongoing dementia trials to identify common biological factors in aging, which they can then try to target.
Alkahest, which was co-founded by Wyss-Coray, takes blood plasma from donors and separates out the part that contains hundreds of proteins the size of known anti-aging proteins. They then infuse this plasma fraction into elderly patients with Alzheimer’s or Parkinson’s disease. Early results suggest that the transfusions are safe and that they improve some of the symptoms of these diseases.
But a number of questions remain before any of these therapies can become practical treatments for normal aging. It’s unclear, for instance, how long the effects of a young-blood transfusion would last, and whether there are side effects. And because transfusions are difficult and because each person’s blood contains a different collection of proteins, Alkahest’s ultimate goal is not to use plasma but to develop drugs that boost these anti-aging proteins. “We just know that proteins are driving a large part of the activity, but we need to get down to the specific proteins and why these proteins are working,” Braithwaite says. “To actually develop the prophylactic therapeutic, it’s going to take 10-plus years.”
However distant that treatment may be, Villeda is encouraged by possibly the greatest contribution of parabiosis to aging research: the idea that normal memory loss is reversible. “Aging is not final; there’s enough reserve in the brain you can reanimate or revitalize it,” Villeda says. Cognitive function, he says, “is just being inhibited or restricted, and all we’re doing is breaking open those restrictions.”


