
In Olfactory System, a Balance of Randomness and Order

In Aldous Huxley’s 1932 novel Brave New World, characters visit “feelies” — multisensory experiences that include performances on a “scent organ,” an olfactory equivalent of a musical instrument. Sadly, despite numerous attempts to bring these sorts of olfactory experiences to life (most hysterically with John Waters’ 1981 film Polyester, which included cued scratch-and-sniff cards), Huxley’s idea remains elusive to this day.
This is in part due to our relative ignorance concerning the relationship between odors and their neural representations. In some ways, our understanding of the olfactory system and of the chemicals that stimulate it lags far behind that of other senses — we don’t even know the basic building blocks of smell. In vision, for example, we know that a small change in the basic physical properties of light, such as its wavelength, will elicit a predictable change in perception — in this case, the color we perceive. For chemicals that have a smell, minor changes in their chemical structure — say, the presence or absence of an extra carbon atom — can give rise to wildly different odor perceptions. Without a grasp of these basic properties of smell, our ability to engineer a device like Huxley’s scent organ remains a pipe dream.
New research published in Nature in July by Sandeep Robert Datta, a neuroscientist at Harvard University and an investigator with the Simons Collaboration on the Global Brain, and his collaborators breathes fresh air into this stinky situation. Stan Pashkovski, a postdoctoral researcher in Datta’s lab, broke down each entry in a library of odorous compounds into a list of its basic features — including molecular weight, polarizability and hydrophobicity — and computed the similarity of pairs of compounds based on these features. Using this similarity measure, Pashkovski was able to select highly similar and highly dissimilar odorous compounds and examine how they were encoded in the brain of a mouse at various stages along the olfactory processing system.
Datta and his team found that, like so many biological systems, the olfactory system seems to use a smorgasbord of computational solutions, some consistent with models developed over the last decade and some reminiscent of classic models developed at the very inception of modern theoretical neuroscience. Taken together, the findings offer the first comprehensive view of how odors are encoded in the brain of an awake animal. Perhaps more importantly, these data have made it possible for Datta and his colleagues to test emerging theories of how information is encoded and transformed by the olfactory system.
That new-theory smell
Even before setting out, Datta had some inkling of how these experiments might turn out. Over the past decade, the olfactory system has become something of a haven for theorists seeking uncharted territory in the brain. “The reason olfaction is interesting is that there is so little initial processing needed,” says Larry Abbott, a theoretical neuroscientist at Columbia University and an SCGB investigator. “Vision goes through dozens of layers that deal with the local structure of images. With olfaction, you get quickly to the business of cognition rather than representation.”
As a picture of the olfactory system’s basic anatomy slowly begins to emerge, so do questions about the organization of the stimuli that activate it, and theorists have jumped on the opportunity to explore new hypotheses about how neural systems might compute. But for Datta, some basic empirical questions remained about how the olfactory system responds to stimuli. “Quite a lot of ink has been spilled speculating about the key computational principles of olfactory processing,” he says. “We were really motivated to find some empirical support for these theories that were, to some extent, outpacing the data.”
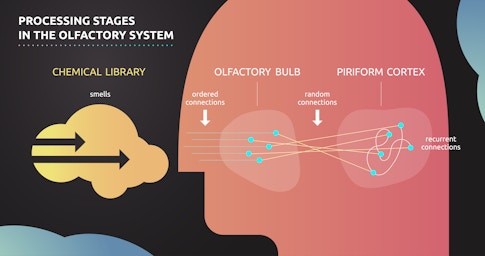
A central theme of these emerging theories is the role of random synaptic connections — connections that do not exhibit a discernible systematic structure at a fine spatial scale. In 2013, Abbott and Richard Axel, also an SCGB investigator at Columbia, identified random connections between peripheral and primary olfactory processing stages in Drosophila. Theorists were intrigued by the discovery of these random connections, in part because they stood in stark contrast to a well-known facet of the olfactory system at that time — its exquisite organization. Nobel prize-winning work by Axel and his postdoctoral researcher Linda Buck had established that olfactory epithelial neurons, the very first stage in olfactory processing, express just one type of olfactory receptor, the molecular machine the cell uses to identify the presence of an odorous chemical. Epithelial neurons with the same receptor send their axons to a common olfactory glomerulus. It turns out that the synaptic connections that emerge from these well-matched glomeruli are random. In other words, the first part of the olfactory system seems tightly structured, and the second part is a random tangle. This is precisely the type of puzzling design principle that has attracted theorists to neuroscience for decades.
One might expect that the brain, with all its sophistication, could come up with something better than random organization. However, a litany of theoretical investigations have determined that random connections have numerous computational advantages. For example, a 2017 Neuron paper by SCGB investigator Ashok Litwin-Kumar and colleagues found that sparse, random connections are fantastic for creating representations that are higher-dimensional than their inputs. High-dimensional representations — where each neuron’s response is strongly independent of every other neuron’s response — are less correlated than the incoming stimuli they represent, making similar representations more dissimilar. Decorrelated representations make it easier to distinguish two similar stimuli, and they vastly increase the computational capacity of a neural system. The fact that random synaptic connections can achieve this feat is nothing short of remarkable.
However, these models have not been universally accepted. “For some time now, detractors of the ‘random connectivity’ view of the olfactory system said that it couldn’t be random, because then you couldn’t generalize,” says Evan Schaffer, an SCGB postdoctoral fellow. Generalization refers to the ability to group similar things — for example, recognizing limes and lemons as citrus fruits. If representations of two similar items were as decorrelated as the random connectivity models predicted — if the representations of limes and lemons were wildly different, for example — then generalization would be challenging. Schaffer sought to address these concerns in a 2018 Neuron paper, which found that the representations following random connections were less correlated than the representations that preceded them, but they were still correlated enough to support generalization. Furthermore, although accurate generalization for a neural system that uses random connections requires many neurons, mice seem to have enough to get the job done.
As exciting as these theoretical investigations were, their conclusions were still based on mathematical models without extensive empirical evidence. Thanks to some key methodological advances, Datta and his colleagues were poised to provide that evidence.
A bouquet of solutions
Armed with knowledge about the chemical similarity of odors, Datta and colleagues used fluorescence imaging to examine how these odors are represented at various processing stages in the olfactory system. They looked at whether similarities in chemical space were conserved in the olfactory bulb, prior to the random connections, and whether they were conserved in the piriform cortex, after the random connections. Examining how correlations were or were not preserved at various stages within the olfactory system would allow Datta to verify or refute the key predictions of the theorists.
The piriform cortex preserved the correlation structure for some pairs of chemicals: Some similar pairs were represented with similar neural activity patterns, and some dissimilar pairs were represented with dissimilar neural activity patterns. But the relationship between neural representations and chemical identity also varied in some very specific ways. Sometimes the piriform cortex represented two odors more similarly than one might expect given the level of similarity of their chemical features. This result was surprising and disagreed with the predictions of random connectivity theories — it suggested that for some pairs of odors, the piriform representation was in fact more correlated than its inputs, not less. What gives rise to these changes in representation in the piriform cortex, and what do they mean for random connectivity models?
It’s possible that the differences exhibited by these specific odor pairs arise in the olfactory bulb and are then passed on to the piriform cortex, a result that would spare random connectivity models from the waste bin. Alternatively, network connectivity within the piriform cortex may modify inputs from the olfactory bulb to make certain odor representations more similar. Such a modification is a hallmark of a long-standing neural network model, auto-associative networks, which are known to address the problem of generalization. Auto-associative networks can be constructed so that similar things become more similar, making it easier for them to be associated with one another. The most famous example of this type of network is the Hopfield network, which can help restore corrupted memories through an auto-associative mechanism. Evidence for auto-associative networks in the piriform cortex would suggest that random connectivity is not the only computational mechanism employed by the olfactory system.
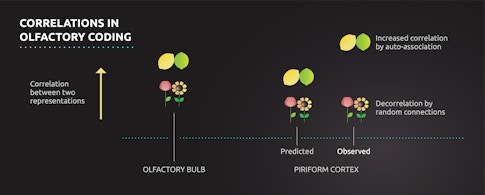
To discriminate between these two possibilities, Datta and colleagues examined how the neurons of the olfactory bulb represent odors. They selectively labeled the part of the olfactory bulb projection neurons in mice that randomly connect to the piriform cortex and found that, for some pairs of odors, the piriform cortex representation does inherit the pairwise correlation structure of the olfactory bulb, albeit with an overall decrease in the magnitude of correlation, consistent with random synaptic connectivity models. But the piriform representation for other pairs of odors differed in key ways from the correlation structure in the bulb, suggesting that its activity patterns are, in fact, also selectively modified, potentially by auto-associative mechanisms. Like so many other biological systems, it seems the olfactory system chose diversity of design over simplicity.
To confirm that an auto-associative process in the piriform cortex produces these enhanced correlation patterns, the authors used genetic techniques to inactivate synaptic transmission within it. This effectively silenced the putative auto-associative mechanisms without removing random feedforward inputs. Doing so increased the similarity between the olfactory bulb and piriform responses for odor pairs that previously showed increased correlation in the piriform, consistent with the existence of auto-associative modification. Moreover, in another set of experiments, Datta found that repeatedly presenting an animal with unrelated odors selectively enhanced the correlation structure in the piriform cortex, consistent with auto-associative grouping of related stimuli. “[Recurrent, plastic connections] are exciting to see and point to a role of piriform cortex in storing and reflecting correlations arising from experience,” Abbott says.
Datta’s results provide strong support for combined processing by random connections and auto-associative modification in the piriform cortex, but questions remain as to whether this is a feature of all olfactory systems or if it’s specific to rodents. Hokto Kazama, an investigator at the RIKEN Center for Brain Science in Japan, and colleagues performed a very similar set of experiments in Drosophila. They did not find evidence supporting auto-associative modification in the mushroom body, the Drosophila equivalent of the piriform cortex. Datta’s results suggest that recurrent connections in the piriform cortex create a representation that’s lower-dimensional and more correlated than what it receives from the olfactory bulb. But Kazama found that the mushroom body creates a higher-dimensional representation than its inputs, which is strikingly consistent with the prediction of random connectivity models. Those results were published online in Neuron in August.
Kazama’s results offer strong support for random connectivity models without auto-associative modification, at least in Drosophila. But as the initial discovery of random connections in the olfactory system has shown, our understanding of anatomy is constantly evolving. Future results could tip the balance between random connections and auto-associative models. For example, scientists know the piriform cortex has recurrent connections, which are critical for auto-association. But whether they exist in the Drosophila mushroom body is an open question. Though the mushroom body seems to lack standard recurrent synaptic connections, ongoing electron micrograph work hints that it has something functionally similar: connections between its cell’s axons. “But we do not know their significance, whether they are excitatory or inhibitory, or even if they are functional at all,” says Litwin-Kumar. “If it turns out they are strong and excitatory it would require rethinking [random connectivity] models.”
Taken together, the findings don’t necessarily invalidate the predictions of random connectivity models but rather suggest a more complex picture of the circuitry of smell in which auto-associative modification and random expansion work together to support olfactory perception. Indeed, these two features might even be a more general theme in the brain, says Schaffer. His work has shown that random connectivity is a simple way to build a good representation of stimuli that can be easily harnessed by other brain regions for additional processing. Then, “modifying the representation at each stage [through auto-association] to better represent what’s important seems likely to be a good idea,” says Schaffer.
Datta hopes to work with theorists to explore how these two computational mechanisms might intersect. He hopes the outcome will be as transformative for olfaction as previous efforts have been for vision. “The development of hierarchical models of vision, in coordination with experimental results, has been such an exciting development in the field of vision,” Datta says. “Our results suggest that developing similar types of models for olfaction makes a lot of sense.”


