Secret to Fruit Fly Attraction Traced to Subtle Electrical Changes
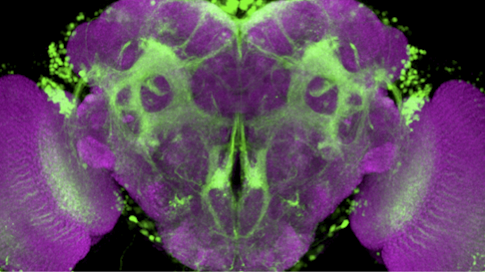
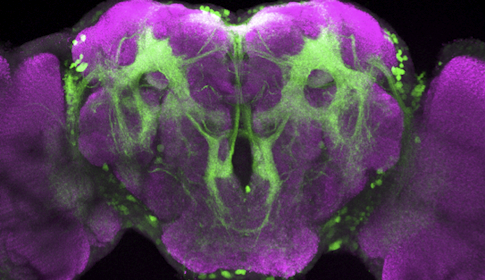
Drosophila melanogaster and Drosophila simulans, two closely related species of fruit fly, have completely different ideas about what makes for an attractive mate. D. melanogaster males are entranced by a pheromone called 7,11-HD, whereas D. simulans males find it repulsive. The difference serves to maintain the separation of the species — both live in the same types of environments, so they need a way to focus mating efforts on their own kind.
Indeed, these kinds of breeding barriers serve an important role across the animal kingdom. But scientists know little about how evolution brings them about. In the fruit fly’s case, both species can accurately detect the pheromone. So why do they react to it so differently?
“Neuroscientists have been working for a long time on how neural processing pathways are altered by individual experience, but we know almost nothing about how the nervous system is shaped over longer time frames” that are the hallmarks of evolution, says Vanessa Ruta, a neuroscientist at Rockefeller University in New York City and an investigator with the Simons Collaboration on the Global Brain (SCGB). “It’s only recently become feasible to explore in a mechanistic way how the same neural circuit is adapted in closely related species to allow for species-specific behavior.”
Ruta’s findings, published in July in Nature, suggest that the species’ different mating preferences are rooted deep in the brain: Minor alterations in the balance of excitation and inhibition in the relevant neural circuit lead to drastic differences in behavior.
“What changed is really subtle,” says Nicolas Gompel, a biologist at Ludwig Maximilian University of Munich in Germany, who was not involved in the research. “It’s not where the information goes but the balance of activation and inhibition that’s particular to the structure of the circuit.”
Fruit fly courtship is an ideal system to study the evolution of neural circuits and behavior because fruit flies’ mating rituals are hard-wired. Males select an appropriate mate, follow the female and sing to her. Scientists have mapped out the circuitry tied to virtually each step of the process in D. melanogaster.
To examine the corresponding circuitry in D. simulans, Ruta and her collaborators first had to modify the molecular tools they use to study D. melanogaster. They could then compare the two species’ neural circuits, a highly unusual feat. “You need a map of both species and to be able to orient yourself in two species at same time,” Gompel says. “This is essentially uncharted territory.”
The simplest explanations for why the two species respond so differently to the same pheromone would be that they sense the molecule differently or route that information to different parts of the brain. But Ruta’s team found no obvious differences in the peripheral nervous system. Nor did the deeper neural circuitry in the two species show major changes. Instead, the researchers found differences in how the signal is propagated through the circuit.
Previous research in D. melanogaster has shown that P1 neurons, which integrate sensory information, play an essential role in courtship. Ruta’s team found that P1 neurons play the same role in D. simulans. Artificially activating P1 neurons triggers courtship behavior in both species, even in the absence of a female.

These neurons receive information from both excitatory and inhibitory sensory cells. Ruta and her collaborators showed that altering the balance of excitation and inhibition across those two populations activates or silences P1 cells. In D. simulans males, the mating pheromone activates the inhibitory pathway, quieting P1 and ultimately stopping the fly from mating. Scientists don’t yet know what molecular or biochemical changes make the circuits behave so differently.
“It was surprising that the change was localized to this one node in the nervous system,” Ruta says. “Maybe this is a flexible point in the system.” Ruta notes that P1 neurons are also modulated by an animal’s individual experience; flies raised in isolation have more active P1 neurons and are more likely to court females. That suggests that the same circuit nodes are flexible at both long evolutionary timescales and over the lifespan of individual animals, she says.
Neuroscientists, including those in the SCGB, are fundamentally interested in how changes in neural circuits lead to changes in behavior. “Evolution is a different lens to think about the relationship between neural architecture and behavior,” Ruta says. “It might tell us how flexibility is engineered into the nervous system and can lead to differences in behavior.”


