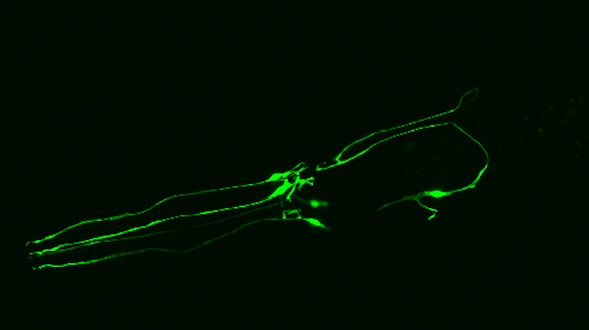
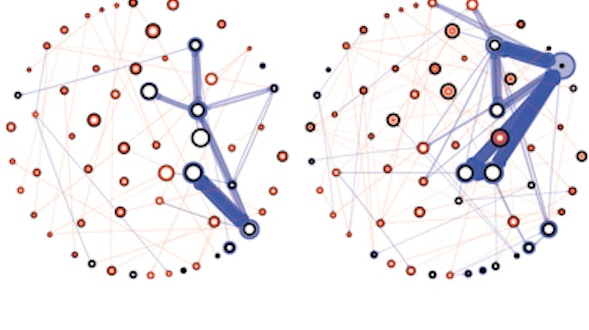
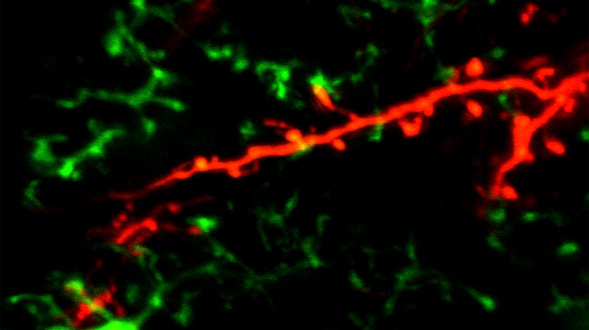
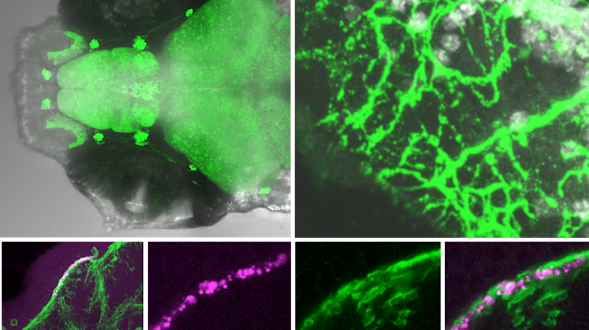
As we age, our ability to learn and remember starts to decline, even in the absence of age-related dementia. We haven’t identified the critical molecular changes that occur in the aging brain or how these changes affect plasticity, the brain’s ability to rewire its connections. To develop interventions to slow or reverse this decline, scientists must first characterize these changes at the molecular and cellular levels and identify the key genes that regulate these processes in the brain. Both of these tasks are challenging. We will leverage the genetic similarities between humans and many different organisms, ranging from worms to fish to mice to nonhuman primates, to examine how these organisms’ ability to learn, a behavioral measure of plasticity, changes with age. This approach can identify a set of core hubs of plasticity in the aging brain that are conserved across species, as well as species-specific changes. We will assess how much these regulators are conserved across different cell types, across individuals of the same species and across species. The promising potential therapeutic targets are those that are shared across species, and using multiple model systems and integrating the information across species will yield information that is greater than the sum of the individual systems.